Newsletter
ALK drug resistant mutations: Challenges for the treatment of lung cancer

Carna Newsletter Vol.15
ALK gene rearrangements are associated with NSCLC
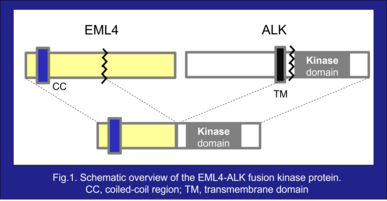
Abnormal anaplastic lymphoma kinase (ALK) fusion genes are present in approximately 5% of patients with non-small cell lung cancer (NSCLC)¹⁾. In ALK fusion proteins, the kinase domain of ALK is fused to the oligomerization domain of partner proteins²⁾ (Fig.1). Although normal ALK protein activation is dependent on binding with its ligand, ALK fusion proteins oligomerize via the oligomerization domain of partner proteins, resulting in constitutive activation of ALK and its downstream pathways, thereby inducing tumorigenesis. EML4-ALK fusion genes were first described in 2007 by Mano et al. at Jichi Medical Univ. (at that time) and are the most common form of ALK rearrangement in patients with NSCLC³⁾.
Disease relapse is driven by ALK drug resistant mutations
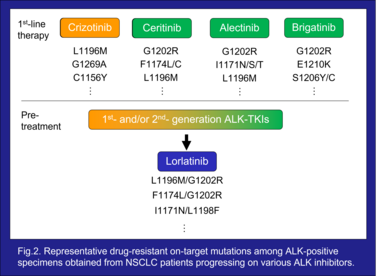
To date, five ALK inhibitors, crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib, have received approval from the FDA and EMA to treat advanced “ALK-positive” NSCLC. Each demonstrated a remarkable tumor response, however the cancer cells rapidly acquire resistance subsequent to treatment, resulting in relapse. One of the tumor’s drug resistance mechanisms is on-target ALK mutation which leads to structural changes in the kinase domain thereby interfering with the binding of the drug⁴⁾⁵⁾⁶⁾⁷⁾ (Fig.2). Secondary ALK mutations were observed in 20-56% of patients progressing on 1st- or 2nd-generation ALK inhibitors⁸⁾.
ALK drug resistant mutations
Crizotinib, the first approved 1st-generation ALK inhibitor in 2011, revolutionized the treatment of ALK-positive NSCLC. Clinical trials revealed that crizotinib was significantly superior to conventional chemotherapy. However, with continued treatment, drug-resistant mutations such as L1196M, G1269A, and C1156Y develop, resulting in tumor relapse and disease progression⁵⁾. Additionally, crizotinib has poor activity against central nervous system (CNS) NSCLC-metastases (e.g., due to poor blood–brain barrier (BBB) penetration), leading to a high incidence of CNS metastases, such as in the brain⁴⁾.
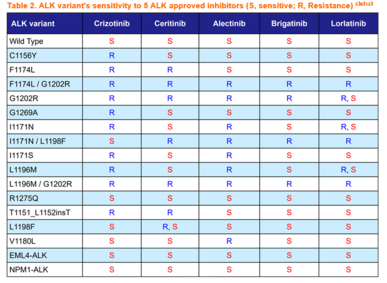
To overcome crizotinib resistance, 2nd-generation ALK inhibitors namely, ceritinib, alectinib, and brigatinib, were developed with improved CNS penetration. Among these, alectinib was associated with approximately 3-fold longer progression free survival (PFS) compared to crizotinib in the first-line treatment of ALK-positive NSCLC in phase 3 clinical trials⁴⁾ and thus it is widely used as a first-line therapy for ALK-positive NSCLC. Despite the highly favorable initial response, the cancer cells soon acquired resistance to all 2nd-generation ALK inhibitors including alectinib where its G1202R and I1171N/S/T mutations, in particular, frequently emerge after the failure of alectinib⁵⁾.
The 3rd-generation ALK inhibitor, lorlatinib, was developed to overcome resistance associated with single mutations caused by treatment with 1st- and 2nd-generation ALK inhibitors. The G1202R mutation is the most common secondary resistant ALK mutant in patients post-treated with 2nd-generation ALK inhibitors, and to date only lorlatinib demonstrates inhibition of G1202R among all approved ALK inhibitors⁴⁾⁵⁾. Yet, even after use of lorlatinib in patients who have already received sequential 1st- and 2nd-generation ALK inhibitors, drug-resistance double mutations emerge⁶⁾⁷⁾. L1196M/G1202R and F1174L/G1202R, among these, are mutations resistant to all approved ALK inhibitors⁹⁾. Therefore, the development of new drugs to overcome these resistance mutations is urgently needed.
New approaches for overcoming resistance to ALK inhibitors
Currently, new-generation ALK inhibitors are being developed to overcome the resistance seen in the approved drugs. For example, TPX-0131 is a compact macrocyclic molecule designed to fit within the ATP-binding pocket and inhibits many key resistance mutations, such as the gatekeeper mutation (L1196M), solvent front mutation (G1202R), and hinge region mutations. In addition, with double mutations such as L1196M/G1202R, for which no effective approved drugs exist, TPX-0131 demonstrated potent inhibition of cell proliferation in cell assays and tumor growth regression in a xenograft tumor model¹⁰⁾. Similarly, NVL-655 also showed potent inhibition of cell proliferation and tumor growth regression against single mutations such as L1196M, G1202R, I1171N and double mutations such as L1196M/G1202R¹¹⁾. TPX-0131 and NVL-655 are currently proceeding to phase 1/2 clinical studies.
Drug repurposing is an alternative approach to finding potential treatments for ALK driven NSCLC. As an example, double ALK mutations containing I1171N/S are resistant to the widely used alectinib and lorlatinib, however, gilteritinib, which has been clinically approved for treating FLT-3 mutant acute myeloid leukemia (AML), was shown to suppress cellular proliferation of Ba/F3 cells expressing these double mutations¹²⁾. This published research suggests that other approved kinase inhibitors with ‘off-target’ ALK activity may be useful in attacking ALK-driven tumors.
As an additional strategy, studies using degraders based on approved ALK inhibitors are also underway. Among them, SIAIS117, a degrader based on brigatinib, showed a better growth inhibitory effect than brigatinib itself on 293T cells expressed with ALK G1202R¹³⁾.
ALK products & services from Carna
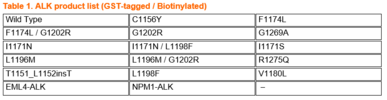
Wild type and 14 mutants of ALK recombinant kinase (shown in the Table 1), all manufactured in-house, are available from Carna with a GST tag, as well as with a single biotin at the N-terminus of the protein. These proteins surely help to reduce your efforts and increase your data quality in your biological assays, including binding assays to investigate your bivalent PROTAC degraders. Assay kits and biochemical activity-based assay services employing our in-house produced active kinases are also ready to support your research.
If you are interested in any mutants not listed on the table below, please feel welcome to contact us at info@kinaselogostics.com.
・Kinase Proteins
・QuickScout Screening Assist™ Mobility Shift Assay Kits
・Activity-based Biochemical Screening/Profiling Assay Services
引用:
- ) Lung. 2020; 198(6): 897-907. Alexander M.
- ) J Clin Oncol. 2018; 36(12): 1199-1206. Lin JJ.
- ) Nature. 2007; 448(7153): 561-6. Soda M. & Mano H.
- ) Front Oncol. 2021; 11: 713530. Pan Y.
- ) Cancer Discov. 2016; 6(10): 1118-1133. Gainor JF.
- ) Cancer Discov. 2018; 8(6): 714-729. Yoda S.
- ) Clin Cancer Res. 2020; 26(1): 242-255. Recondo G.
- ) Cancer Discov. 2017; 7(2): 137-155. Lin JJ.
- ) EBioMedicine. 2019; 41: 105-119. Okada K.
- ) Mol Cancer Ther. 2021; 20(9): 1499-1507. Murray BW.
- ) Nuvalent website, Corporate Overview September 7, 2022.
- ) Nat Commun. 2021; 12(1) :1261. Mizuta H. & Katayama R.
- ) Eur J Med Chem. 2020; 193: 112190. Sun N.
Further news
-
Newsletter
Cancer treatments using inhibitors of CDK, a cell cycle regulator
Read moreCarna Newsletter Vol.16
-
Technical Note
DGKα and DGKζ are key targets for cancer immunotherapy
Read moreCarna Newsletter Vol.14
-
Newsletter
The potential of PIKfyve inhibitor as a treatment of amyotrophic lateral sclerosis (ALS)
Read moreCarna Newsletter Vol.13
