Newsletter
Kinase Inhibitors as Potential Therapeutic Agents for COVID-19

Carna Biosciences Newsletter Vol. 11
Follow-up to Carna Biosciences Newsletter Vol.7, “Kinase Inhibitors in the Fight against COVID-19”
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the pandemic disease which was named COVID-19 (coronavirus disease 2019). Due to its high morbidity and mortality, there remains an urgent need for effective pharmacologic therapies to minimize mortality and decrease viral shedding and subsequent transmission around the world.
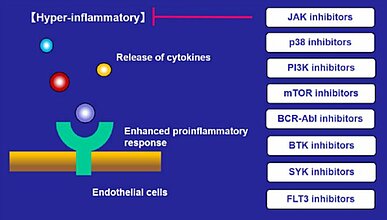
In certain instances, infection by SARS-CoV-2 can cause acute respiratory distress syndrome (ARDS), one of COVID-19’ s leading causes of mortality. The cytokine storm caused by the release of proinflammatory cytokines after infection, is the most prominent feature of ARDS. ICU COVID-19 patients were reported to have plasma levels of several cytokines including IL-2, IL-10, G-CSF higher than non-ICU patients (1). Additionally, blood levels of IL-6 were found to be elevated in non-survivors compared with survivors. IL-6 is associated with death and appears to be one of the predictors of mortality (2) (3).
JAK1/JAK2 inhibitor, Baricitinib
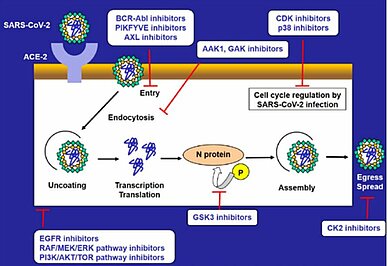
Using artificial intelligence (AI) algorithms to search approved drugs capable of inhibiting both the inflammatory damage and infectivity associated with SARS-CoV-2, baricitinib (an oral JAK1/JAK2 inhibitor) approved for the treatment of rheumatoid arthritis (RA) was predicted to be a potential treatment for COVID-19 (4) (5). Baricitinib inhibits signaling of cytokines implicated in severe cases of COVID-19, and reduces IL-6 plasma levels in RA patients (6). Additionally, baricitinib demonstrated potent inhibition of AAK1 and GAK, members of the NAK family which regulate clathrin-mediated endocytosis, and reduced SARS-CoV-2 infectivity in primary human liver spheroids as an anti-viral activity (6).
Clinical data showed the potential role of baricitinib to treat certain COVID-19 hospitalized patients and is now authorized under an Emergency Use Authorization (EUA) by the FDA in adult patients and pediatric patients 2 years of age or older requiring supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) (7) (8) (9).
BCR-Abl inhibitor, Imatinib
It is also reported that imatinib, a BCR-Abl inhibitor, prevents the cell entry of SARS-CoV-2 pseudovirus and has immunomodulatory effects which include attenuation of proinflammatory cytokine release (10) (11). In a clinical trial of treatment for COVID-19, reduction in mortality at 28 days and duration of mechanical ventilation in patients given imatinib were observed, compared with the placebo-group (12). This result provides an expectation of a clinical application repurposing imatinib to treat COVID-19, after further studies.
Other kinase inhibitors
In addition to the described JAK and BCR-Abl inhibitors, other kinase inhibitors with anti-viral activity against SARS-CoV-2, or immunomodulatory effects such as cytokine suppression have been reported (Fig.1,2) (13) (14) (15) (16). With the goal of finding more treatment options for SARS-CoV-2, clinical trials with CK-2, BTK, SYK, PI3K or mTOR inhibitors (13) (16) and pre-clinical tests with CDK inhibitors are currently ongoing. Many kinase inhibitors have been effectively used in the clinic as anticancer agents, and now their usefulness in infectious disease such as COVID-19 has attracted attention.
All kinase products including those targeted by the kinase inhibitors currently anticipated to be therapeutic agents for COVID-19 are manufactured in-house at Carna Biosciences and available. In addition, Carna offers a biochemical activity-based assay service using these enzymes and a cell-based binding assay service to assist you in your kinase inhibitor drug discovery. Below is an overview of our tool kit for this, and for finding treatments for other diseases.
- GST-tagged (includes some HIS and tag free) Kinase Protein Products
- Biotinylated (DYKDDDK tagged) Kinase Protein Products
- Activity-based Biochemical Screening/Profiling Assay Services
- NanoBRET™ TE Intracellular Kinase Assay Services
We welcome your comments on this newsletter and any questions about our products. Please feel welcome to contact us.
References:
- Lancet. 2020; 395(10223): 497-506. Huang C.
- Lancet. 2020; 395(10229): 1054-1062. Zhou F.
- Intensive Care Med. 2020; 46(5): 846-848. Ruan Q.
- Lancet. 2020; 395(10223): e30-e31. Richardson P.
- Lancet Infect Dis. 2020; 20(4): 400-402. Stebbing J.
- EMBO Mol Med. 2020; 12(8): e12697. Stebbing J.
- N Engl J Med. 2021; 384(9): 795-807. Kalil AC.
- Eli Lilly’ s news release, July 29, 2021.
- Eli Lilly’ s news release, August 3, 2021.
- bioRxiv. 2020. N Mulgaonkar.
- Bio Blood Marrow Transplant. 2018; 24(2): 267-275. Marinelli Busilacchi E.
- Lancet Respir Med. 2021; 9(9): 957-968. Aman J.
- J Med Chem. 2021. Pillaiyar T. Chen CZ.
- Cell. 2020; 182(3): 685-712.e19. Bouhaddou M.
- Mol Cell. 2020; 80(1): 164-174.e4. Klann K.
- Blood adv. 2021; 5(3): 913-925. Jacobs CF.
Further news
-
Newsletter
Cancer treatments using inhibitors of CDK, a cell cycle regulator
Read moreCarna Newsletter Vol.16
-
Newsletter
ALK drug resistant mutations: Challenges for the treatment of lung cancer
Read moreCarna Newsletter Vol.15
-
Technical Note
DGKα and DGKζ are key targets for cancer immunotherapy
Read moreCarna Newsletter Vol.14
