Newsletter
Targeted Degradation of Non-catalytic Kinases New Drug Discovery Options

Carna Biosciences Newsletter Vol. 10
The announcement that Kymera Therapeutics, a company pioneering targeted protein degradation, entered into a strategic collaboration with Sanofi to develop and commercialize first-in-class protein degrader therapies targeting IRAK4 in patients with immune-inflammatory diseases highlights the growing interest in clinical applications of small molecule mediated kinase degradation. Kymera received $150 million in cash up front and may potentially receive at least $2 billion in this key strategic partnership, including sales milestones and royalty payments.
Most of the protein degraders currently under development are heterobifunctional molecules which contain one moiety that binds a desired target protein and another that binds an E3 ligase, joined by a linker. Protein degrader-induced proximity results in ubiquitination of the target followed by its degradation by the proteasome. This transformative new modality is expected to open a new chapter in drug discovery targeting kinases for which the development of clinical inhibitors has been difficult. One such example is IRAK4, which has a non-catalytic function independent of kinase activity in addition to a catalytic function as a kinase.
Scaffolding function
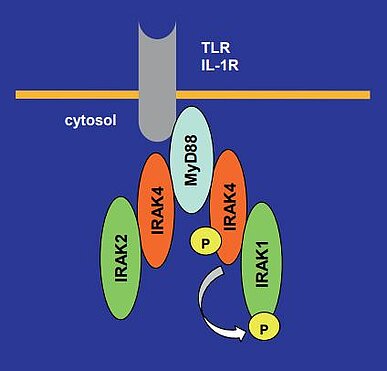
IRAK4 is involved in the downstream regulation of Toll-like receptor (TLR) and IL-1 receptor (IL-1R) pathways. IRAK4 not only trans-autophosphorylates to phosphorylate IRAK1 but also interacts with IRAK1, IRAK2 and MyD88 as a scaffolding protein independent of kinase activity (Fig.1) (1). In this way, IRAK4 has an important role in promoting the production of pro-inflammatory cytokines. Several reports have shown that inhibition of IRAK4 kinase activity alone could not sufficiently suppress the TLR/IL-1R signaling in several cell types, which indicates that the IRAK4 scaffolding function is important in some cases (2) (3).
Allosteric regulatory function
Aside from scaffolding functions, allosteric regulation is another non-catalytic function that activates binding partners by inducing a conformational change. For example, in the RAF family of kinases, A-RAF, B-RAF and C-RAF form homodimers or heterodimers and allosterically activate their binding partners independent of kinase activation. In the case of RAF kinases, only the active kinase conformation is compatible with allosteric transactivation of kinases in RAF dimers (4). Several ATP-competitive inhibitors of RAF stabilize the active conformation of the kinase where the αC-helix is rotated toward the active site (αC-helix IN). As a result, inhibitor-bound RAF molecules can dimerize with and allosterically activate apo RAF molecules, dependent upon RAS, which attenuates the inhibitory effect (5) (6). Thus, there are cases where conventional ATP-competitive inhibitors are unable to sufficiently suppress the functions of kinase targets simply because the targets have a kinase-activity-independent function.
Significant research is currently underway on protein degraders targeting kinases that have a non-catalytic function independent to kinase activity and for which conventional small molecule inhibitors have not been successful in clinical trials. FAK is an example where an important scaffolding function exists independently of kinase activity. It has been reported that a protein degrader targeting FAK significantly inhibits its downstream signaling, as well as impairing cell migration and invasion to a greater extent than defactinib, a conventional small molecule inhibitor (7). In addition, one report showed that a protein degrader targeting AURORA-A, which binds to N-MYC independently of kinase activity and protects N-MYC from degradation by the proteasome, caused S-phase arrest, which was not observed upon kinase inhibition (8). Targeting of kinases that have a non-kinase catalytic function by protein degradation creates the potential to discover new therapeutic opportunities.
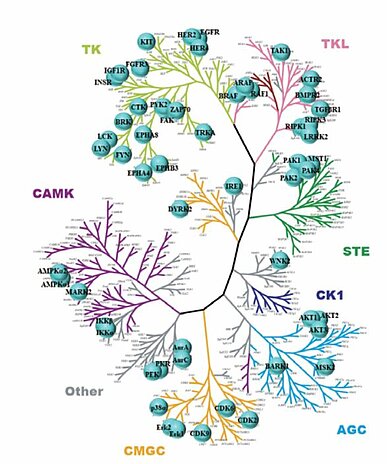
In addition to the kinases noted in this newsletter, it has been reported that more than 50 kinases have non-catalytic functions (Fig.2) (4). Drug discovery programs targeting this function may ultimately expand overall opportunities for clinical success using this therapeutic modality.
All kinases and related products sold by Carna, are manufactured in-house and delivered to you after stringent quality control, identity confirmation and activity assessment. In addition to GST tagged kinases, Carna produces a wide selection of biotinylated kinases labeled with a single biotin molecule at the N-terminus. Our extensive portfolio of biotinylated kinases can be utilized not only in biochemical activity assays, but also for SPR data acquisition, binding assays using TR-FRET, AlphaScreen/AlphaLISA and other applications.
If you are interested in any kinase or kinase-related product or service that is not seen on our website, please feel welcome to contact us.
References:
- Nat Rev Drug Discov. 2021; 20(1): 39-63. Zarrin AA
- J Biol Chem. 2004; 279(25): 26748-53. Qin J.
- J Immunol. 2011; 186(2): 1279-88. Chiang EY.
- Structure. 2016; 24(1): 7-24. Kung JE.
- Nature. 2010; 464(7287): 431-5. Hatzivassiliou G.
- Nature. 2010; 464(7287): 427-30. Poulikakos PI.
- J Am Chem Soc. 2018; 140(49): 17019-17026. Cromm PM.
- Nat Chem Biol. 2020; 16(11): 1179-1188. Adhikari B.
Further news
-
Newsletter
Cancer treatments using inhibitors of CDK, a cell cycle regulator
Read moreCarna Newsletter Vol.16
-
Newsletter
ALK drug resistant mutations: Challenges for the treatment of lung cancer
Read moreCarna Newsletter Vol.15
-
Technical Note
DGKα and DGKζ are key targets for cancer immunotherapy
Read moreCarna Newsletter Vol.14
