Newsletter
Targeted Protein Degraders

Carna Biosciences Newsletter Vol. 3
This year marked the exciting start of clinical trials for a first in class protein degrader targeting androgen receptor for the treatment of prostate cancer. Arvinas, Inc., is now set to initiate the second clinical program of their PROTAC™ (proteolysis-targeting chimaera) protein degrader for the treatment of patients with locally advanced or metastatic ER positive/HER2 negative breast cancer (targeting estrogen receptor). Increasingly, targeted protein degraders, named PROTAC™s, Degronimids, or SNIPERs, are anticipated to create a paradigm shift in drug discovery by targeting proteins previously thought to be undruggable by small molecules.
Funcrtion of targeted protein degraders
The concept of targeted protein degraders was first introduced in the patent literature in 1999 (1), and a PROTAC™ was published in 2001 (2). A targeted protein degrader is a bifunctional compound which consists of an E3 ubiquitin ligase (E3) binding domain and a target protein binding domain joined by a linker. The protein targeted by the protein degrader is ubiquitinated by the E3 ligase and thus marked for degradation by the proteasomal system. This modality potentially unlocks new possibilities for discovering novel drugs targeting proteins that are difficult to target or have drug resistance mutations. For example, IRAK4 and RIPK, which are known to be involved in cancer and immune diseases, have been difficult to target owing to their dual functions. Both proteins have a catalytic function as a kinase in addition to a kinase-independent role as a scaffolding protein. No traditional small molecule compounds have successfully inhibited IRAK4 and RIPKs dual functions simultaneously. Degradation of these proteins induced by targeted protein degraders offers an encouraging potential approach to achieve such simultaneous inhibition. In fact, research and development (R&D) of targeted protein degraders targeting IRAK4 and RIPK is currently in progress (3).
History of targeted protein degradation
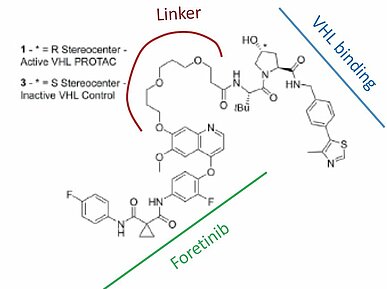
Targeted protein degradation has an interesting history and has been making a quick advancement in drug discovery. Craig M. Crews, Ph.D. of Yale University, a pioneer of targeted protein degrader technology and founder of Arvinas, has worked to optimize the two parts of the bifunctional PROTAC™. The Crews group synthesized two molecules similar to foretinib, a promiscuous kinase inhibitor that binds to 133 different kinases, and linked them to each of two types of E3 ubiquitin ligase-recruiting molecules. One E3 recruiter is a CRBN-recruiting PROTAC™ and another is a VHL-recruiting PROTAC™ (4) (Fig.1).
The investigators measured degradation levels of each kinase induced by these compounds. To their surprise, degradation levels correlated with the stability of the ternary complex between E3 and the target kinase mediated by the PROTACTM irrespective of the binding affinity of foretinib and the PROTAC™ to the kinase. Furthermore, they discovered that many kinases display different degradation levels towards the VHL-bound PROTAC™ and the CRBN-bound PROTAC™, indicating that there is an optimal combination of target kinase and target E3 (4).
Detection of a targeted protein degraders
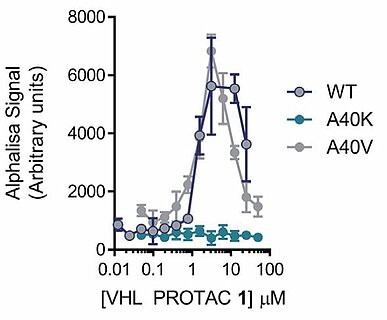
The most well-known method for detecting a ternary complex is a combination of immunoprecipitation using tags or antibodies and Western Blotting. However, the many technical steps in this method make large-scale evaluation difficult. AlphaScreen™ / AlphaLISA™ assay systems from Perkin Elmer are examples of assay platforms designed to overcome this obstacle and facilitate higher throughput. These assays allow detection of a targeted protein degrader binding to a target protein and E3 through the use of two special beads which emit light when they are brought into proximity. This system has already been employed in PROTAC™ R&D as an easy and quantifiable ternary complex detection method (4) (Fig.2).
Carna’ s biotinylated kinases and GST fusion kinases (5) are compatible with AlphaScreen™ / AlphaLISA™ assays and are highly recommendedas having superior quality as purified proteins. These can be easily used in assays using streptavidin or glutathione-coated beads for data acquisition.
References:
- US Patent US6306663B1 1999 J Kenten, S Roberts.
- Proc Natl Acad Sci USA 2001; 98(15): 8554–8559. Sakamoto KM & Crews CM.
- Nat Rev Drug Discov. 2019; 18: 237-239. Mullard A.
- Cell Chem Biol. 2018; 25(1):78-87. Bondeson DP & Crews CM.
- PerkinElmer, Technical note “Development of an AlphaLISA MEK1 Kinase Assay Using Full-Length ERK2 Substrate” , Jeanine H.
Further news
-
Newsletter
Cancer treatments using inhibitors of CDK, a cell cycle regulator
Read moreCarna Newsletter Vol.16
-
Newsletter
ALK drug resistant mutations: Challenges for the treatment of lung cancer
Read moreCarna Newsletter Vol.15
-
Technical Note
DGKα and DGKζ are key targets for cancer immunotherapy
Read moreCarna Newsletter Vol.14
