Newsletter
The importance of understanding the activity of compounds on RAF dimerization in BRAF inhibitor drug discovery

Carna Biosciences Newsletter Vol. 12
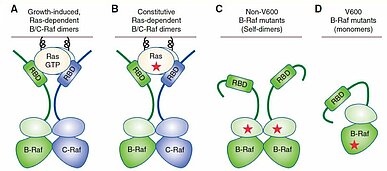
RAF protein kinases (ARAF, BRAF and CRAF) are key components of the RAS-RAF-MEK-ERK signaling cascade. An upstream signal such as from a receptor tyrosine kinase allows RAS to convert to the active GTP-bound form. The GTP-bound RAS binds RAF proteins, inducing RAF dimerization which then activates the downstream MEK-ERK signaling (1) (Fig.1).
There is great interest in BRAF inhibitors, as BRAF is downstream of activated RAS mutants, but also found mutated in 8% of human tumors and particularly in ~60% of melanomas. BRAF [V600E] has been detected in greater than 90% of BRAF-mutant tumors and acts as a monomer, liberated from upstream activation. Three ATP-competitive RAF inhibitors, vemurafenib, dabrafenib and encorafenib, show remarkable clinical efficacy in patients with BRAF [V600E/K] melanoma and received FDA approval for the treatment of this disease. Yet, despite their effectiveness, continuous administration leads to drug resistance and tumor relapse. Moreover, it is reported that vemurafenib and dabrafenib often produce serious side-effects among these, cutaneous squamous cell carcinoma (2). Recent research has revealed that these undesirable events including drug resistance and side-effects are due to ERK activation caused by RAF dimerization, after drug treatment (3).
TypeⅠ1/2 RAF inhibitors (such as vemurafenib, dabrafenib and encorafinib) effectively inhibit the activity of the BRAF V600 mutant monomer but are unable to effectively inhibit the activity of RAF dimer, as the binding of the inhibitor to one monomer of a RAF dimer reduces the affinity of the subsequent inhibitor binding to the other monomer (negative cooperativity) (3). The result of an increase in RAF dimerization is the likely cause of the drug resistance in patients who have continued administration of type Ⅰ1/2 RAF inhibitors. Among the causes of the drug resistance are RAS activation (by RAS mutation or overexpression of the upstream receptor tyrosine kinases) (4), or the expression of splice variants of BRAF [V600E] which can be dimerized independently of RAS activation (5). Additionally, vemurafenib and dabrafenib can induce RAF dimerization, causing post-treatment cutaneous squamous cell carcinomas in BRAF-wildtype cells where RAS is active (so-called, paradoxical activation) (2) (3).
Thus, preventing RAF dimerization is an effective strategy for the development of new, next generation inhibitors with improved safety and durable efficacy, which underlines the value of relevant cell assays that can identifying the dimer formation.
Next-generation RAF inhibitors including typeⅡ pan-RAF inhibitors and paradox-breakers are currently undergoing clinical trials. TypeⅡ pan-RAF inhibitors target both RAF dimers and RAF monomers at similar potencies. However, these inhibitors strongly induce RAF dimerization by locking RAF in αC-helix IN conformation and drive paradoxical activation, although minimally. On the other hand, a paradox-breaker, namely PLX8394, pushes the C-terminal end of the αC-helix in BRAF and CRAF out, thereby perturbing the cross-dimer interactions of BRAF homodimers and BRAF/CRAF heterodimers, resulting in very low levels of paradoxical activation in these inhibitor types (6) (7).
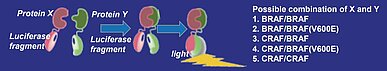
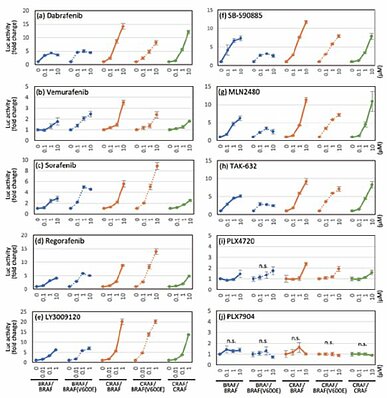
Carna Biosciences’ split luciferase complementation assay technology was used to develop a RAF dimerization assay (Fig.2), and measure RAF inhibitor potency including typeⅠ1/2 RAF inhibitors (dabrafenib, vemurafenib, PLX4720), typeⅡ pan-RAF inhibitors (LY3009120, MLN2480, TAK-632) and paradox-breaker (PLX7904) in inducing the dimerization of 5 distinct RAF isoform pairs, namely BRAF/BRAF, BRAF/BRAF [V600E], CRAF/BRAF, CRAF/BRAF [V600E], and CRAF/CRAF (Fig.2). The results were consistent with previous reports and were published in Scientific Reports (2) (Fig.3).
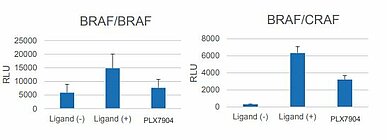
Carna Biosciences offers this RAF dimerization assay as a service. This simple, rapid and quantitative assay makes it possible to detect the potencies of your compounds in inducing RAF dimerization. It can be a useful tool in drug screens to develop RAF inhibitors with minimal or no effect on dimerization and/or adverse events such as paradoxical activation or drug resistance. Additionally, quantification of the inhibitory activity of your compounds in an assay where RAF dimerization is induced by LY3009120 is also possible (Fig.4).
Carna Biosciences also offers ELISA kits targeting BRAF, BRAF [V600E] and CRAF (RAF1) for purchase to readily generate your own data in house.
If you are interested in Carna Biosciences’ products & services, including this RAF dimerization assay and ELISA assay kits, please feel welcome to contact us.
References:
- Br J Cancer. 2018; 118(1):3-8. Durrant DE.
- Sci Rep. 2019; 9(1): 636. Miyamoto K.
- Nat Rev Cancer. 2017; 17(11): 676-691. Karoulia Z.
- Nature. 2010; 468(7326): 973-7. Nazarian R.
- Nature. 2011; 480(7377): 387-90. Poulikakos PI.
- Biochem Soc Trans. 2021; 49(1): 237-251. Cook FA.
- Nat Med. 2019; 25(2): 284-291. Yao Z.
Further news
-
Newsletter
Cancer treatments using inhibitors of CDK, a cell cycle regulator
Read moreCarna Newsletter Vol.16
-
Newsletter
ALK drug resistant mutations: Challenges for the treatment of lung cancer
Read moreCarna Newsletter Vol.15
-
Technical Note
DGKα and DGKζ are key targets for cancer immunotherapy
Read moreCarna Newsletter Vol.14
